Arrange the Following Elements in Order of Increasing Metallic Character
Ti Ra Sr Fr Mn-2. For example the order of increasing metallic character is P Si Be Mg Na.

Metallic Character Trend On The Periodic Table
Arrange the following elements in order of increasing metallic character.

. Clear All cesium LEAST Metallic sodium Second LEAST Metallic potassium Second MOST Metallic. Arrange the following in the increasing order of their metallic character. P block metals have the lowest metallic character.
Cesium which is located just above Francium on the periodic table is the natural product with the strongest metallic character. As we move across the period the metallic character decreases as the there is an increase in nuclear charge and the atomic radius decreases too. Na has 1 magnesium has 2 aluminium has 3 and Chlorine has 7 electrons in its valence shells.
Arrange the following elements in order of increasing metallic character. So the overall order will be. Arrange the following elements in the increasing order of metallic character.
Alkaline earth metals have the next highest metallic character. Least metallic Second Least metallic Second Most Metallic Most Metallic. Least metallic Second Least metallic Second Most Metallic Most Metallic.
Arrange the following elements in order of increasing metallic character. ANSWERS- Correct order of increasing metallic character. Arrange the following elements in the increasing order of their metallic character Mg Ca K Ge Ga Answer.
Chlorine and oxygen are the most electronegative elements. Metallic character increases as one moves down the group because there is an increase in atomic size. Ge Ga Mg Ca K.
With the help of the metallic character trend we can observe the non-metallic character of elements as while moving left to right in a period the non-metallic character increases and on moving down the group the non-metallic character decreases. Tc Hf Ba Nb Cd-3. Clear All lead LEAST Metallic Second LEAST Metallic barium Second MOST Metallic thallium MOST Metallic cesium Arrange the following elements in order of increasing metallic character.
Using periodic trends arrange the following elements in order of increasing atomic radius. Arrange these elements in order of increasing metallic character. Ra Sb Cd Cl La Se.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Arrange the following elements in order of increasing metallic character. Fr Sb In S Ba Se.
By signing up youll get. Arrange the following elements in order of increasing metallic character. The correct order is Ge Ga Mg Ca K.
Clear All lithium LEAST Metallic rubiuium Second LEAST Metallic Second MOST Metallic potassium MOST. Chemistry questions and answers. Arrange the following elements in order of increasing metallic character.
Ti Ra Sr Fr Mn. Alkali metals are the most electropositive elements and have maximum metallic elements. Re Co Db Ga W.
Mg P Si Na Be. In a group on moving from top to bottom the metallic character increases. Arrange the following elements in order of increasing metallic character.
Arrange the following elements in the increasing order of their metallic character. Clear All strontium LEAST Metallic beryllium Second LEAST Metallic magnesium Second MOST Metallic calcium MOST Metallic Arrange the following elements in order of. Clear All boron LEAST Metallic beryllium Second LEAST Metallic carbon Second MOST Metallic nitrogen MOST Metallic Arrange the following elements in order of increasing metallic character.
In periodic table- Metallic character decr View the full answer. Arrange the following elements in the increasing order of their metallic character Mg Ca K Ge Ga. Arrange the following elements in order of increasing metallic character.
The metallic character of an element is defined as the easiness of its atom is. Re Co Db Ga W-. Arrange the following elements in order of their increasing metallic character NaSiClMgAl.
Si Be Mg Na P. Metallic character increases down a group and decreases along a. Write your answers in your notebookon a separate sheet of paper.
Si Be Mg Na P. Tc Hf Ba Nb Cd-. Metallic characteristic decreases across a period on moving right.
Hence Sodium shows maximum metallic characters followed by Magnesium aluminium and chlorine shows non-metallic properties. Arrange the following elements in the order of the their increasing non-metallic character Li O C Be F. 1 Barium Thallium Lead Bismuth 2 Strontium Calcium Magnesium Beryllium 3 Barium Strontium Calcium Magnesium EXPLANATION.
So the correct answer is Option C. In a period on moving from left to right the metallic character decreases.

How Do You Arrange Elements In Order Of Increasing Metallic Character

Which Of The Following Set Of Elements Is Written In Order Of Their Increasing Metallic Character

Introduction To Molecular Geometry Molecular Geometry Molecular Geometry
D Block Elements Iit Jee Study Material With Properties And Examples
Cbse Mcqs For Class 10 Science Chapter 5 Periodic Classification Of Elements

Neon S Melting Point Is 248 67 C Melting Point Boiling Point Atomic Number
Metallic Character The Periodic Table Of Elements

Electrochemical Series Definition Chart Applications Reduction Potential Redox Reactions Reducing Agent

Periodic Table The Basis Of The Periodic System Britannica

Metallic And Nonmetallic Character Chemistry For Non Majors
Metallic Character Of Transition Metals Transition Element

Arrange The Following Elements In Increasing Order Of Metallic Character Na Mg Al K Brainly In

Increasing Order Of Metallic Character Will Be Youtube

Plushees Nintendo Ds 2008 For Sale Online Ebay Nintendo Ds Ds Games Nintendo Ds Games

Alkyl Halides And Elimination Reactions Organic Reactions Reactions Functional Group

Neon S Melting Point Is 248 67 C Melting Point Boiling Point Atomic Number
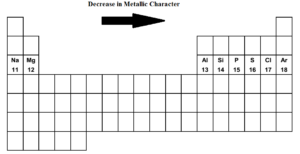
Metallic Character Of Third Row Elements Meaning Trend Factors Affecting
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

Comments
Post a Comment